Hi,
I am trying to get a unit cell of Al2O3. I read that the unit cell should contain 30 atoms, and in r3c style. However when I import the crystal I get cartesion coordinates below for only 10, why is the unit cell like this? Thanks very much
Al 3.88809 2.03610 1.39213
O 6.32203 4.36710 0.98037
Al 1.58809 0.83165 0.56862
Al 7.06427 3.69939 2.52937
Al 9.36427 4.90385 3.35288
O 5.65427 3.77342 3.71366
O 7.19033 1.89660 2.16859
O 4.63033 1.36839 2.94112
O 5.29809 1.96208 0.20784
O 3.76203 3.83890 1.75291
Also, as a follow up. When I try to build Fe2O3 from import crystal I get this:
For 4 Fe atoms I have 12 O atoms. This is not a 2Fe to 3O ratio? Why are these not correct?
Fe 1.21963 0.63869 0.43669
O 3.77522 3.15992 0.00000
Fe 4.58812 2.40269 1.64278
Fe 10.39587 5.44407 3.72224
Fe 7.02737 3.68007 2.51615
O 3.09275 2.44992 2.94452
O 4.74752 0.47292 1.21441
O 6.49022 1.73814 2.07947
O 5.12527 3.51430 3.29387
O 5.80775 3.87170 0.86506
O 4.74752 1.30324 0.00000
O 3.09275 3.63284 1.21441
O 3.77522 1.14668 2.94452
O 5.12527 4.34462 2.07947
O 6.49022 2.56846 0.86506
O 5.80775 2.21106 3.29387
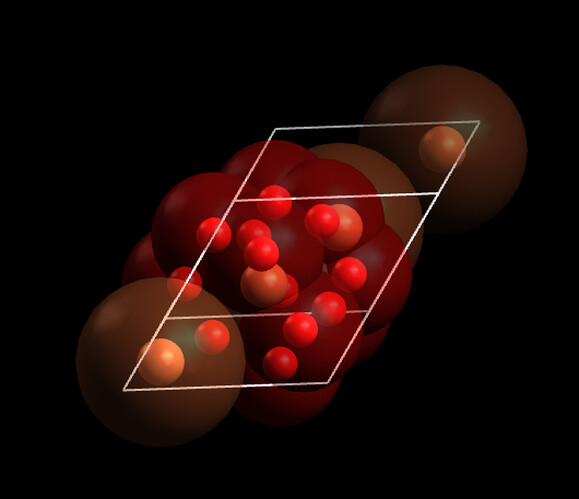
You need to understand how atoms are counted in unit cells. Atoms on cell boundaries count as a fraction. Consider the \ce{Fe2O3} example:
Note that many of the oxygen atoms fall at cell boundaries (two in the picture above). These would count as 1/2 an oxygen each in the formula.
There are other examples that can be found with a search, e.g., at Chemistry Stack Exchange.
Thanks for the response and image!
To be sure, does this mean that I am safe to use this unit cell and replicate it to get a supercell? I am planning on exporting it as a pdb, will the pdb file capture the fact that some of these atoms are half oxygen.
As opposed to the primitive until cell, is there a way to get the conventional unit cell? My ultimate goal is to get these as a data file for lammps, and I will use VMD to write the lammps data file from the pdb. Just want to make sure I am not introducing error anywhere.
To be clear, where in avogadro does it show that only half of these oxygen atoms are being counted. When I replicate this primitive unit cell will it be accurate? Is there a way to get the 30 atom conventional unit cell?
When an atom is on a unit cell plane, it “counts” as half in one unit cell and half in the other - it’s a matter of simple geometry (i.e., half of the atom is in one unit cell and half is in another).
If an atom is on a unit cell “corner” then the volume is shared by 8 unit cells - again from simple geometry. (Neither material you mention has an atom at a corner.)
Yes, if you replicate the unit cell to generate a supercell, it will produce the correct material - that’s the principal of translational symmetry.
I don’t know what you mean by the 30-atom conventional unit cell for \ce{Al2O3}. If you’re not happy with the setting provided with Avogadro, you can also get CIF files off the MaterialsProject.org website, e.g. \alpha-\ce{Al2O3} You can explore the crystal structures using their online Materials Explorer.