hello. I am currently trying to obtain simulated thermodynamic properties of PFAS compounds and its possible cleaved products in different bonds.
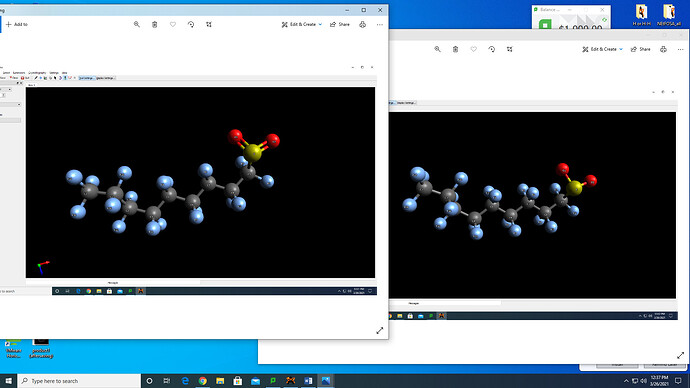
Recently, I have drawn one of the products of a PFAS compound (N-EtFOSA; Sulfuramid). I am interested in the corresponding S-N BDE. I have drawn the products after in which the S-N would be cleaved and would like to consider the product to have an unpaired electron (i.e., radical). I initially drew the cleaved product for one of the moieties that corresponds to having the sulfur (as shown in the first picture.
However, after saving the file and reopening the xyz file, the double bond for the S=O disappears and they are automatically converted into single bonds. How can I preserve the double bond? I think having the double bond could be crucial for vibrational frequency measurements as the double bond would be more limited for molecular rotation compared to single bonds.I will like to run the radical molecule through ORCA with considering multiplicity of 2 by running the double bond added moiety.
JHK