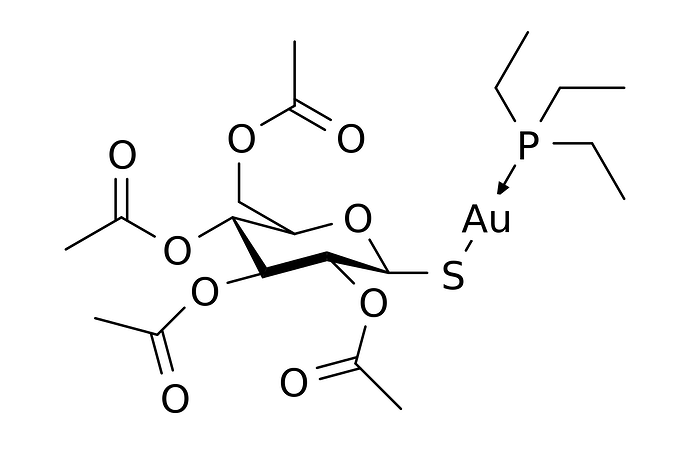
Is there any way to represent Coordinate Covalent Bonds in our models? I’m building the model for Auranofin, and I’m not sure how to represent the CCB between the Gold atom and the Phosphorus atom.
By now, I recommend to use just a plain covalent bond between the phosphine ligand and Au. Indeed, it is the same approach you already engage between Au an the thiolate.
This would follow rule GR 1-7-1 in the recommendations compiled by Brecher. It equally is the pattern generally used in the brief guide to the Red Book (including its handy four-page abstract) to illustrate inorganic nomenclature of coordination compounds, which was published a few years later. All three documents are available as open access.
(1) Brecher, J. Graphical Representation Standards for Chemical Structure Diagrams (IUPAC Recommendations 2008). Pure Appl. Chem. 2008, 80, 277–410. https://doi.org/10.1351/pac200880020277.
(2) Hartshorn, R. M.; Hellwich, K.-H.; Yerin, A.; Damhus, T.; Hutton, A. T. Brief Guide to the Nomenclature of Inorganic Chemistry. Pure Appl. Chem. 2015, 87, 1039–1049. https://doi.org/10.1515/pac-2014-0718.
There have been requests for dashed bonds, for example, but I agree 100% with @Thomas - people will debate dative / agostic / coordinate bonds for ever. That’s fine. Philosophical debates are good. (What is the nature of a bond.)
In this case, I think you want to emphasize the shape / identity of the molecule, so a “regular” covalent bond between the Au and P atoms is warranted.
Thank you both for the clarifications.