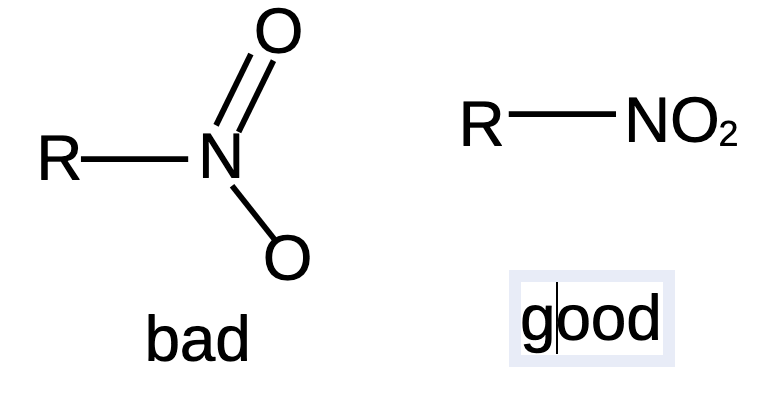
Would you please consider adding condensed group formula for the “nitro” group, instead of one where the bonds are drawn out? The latter is too clumsy for drawing larger molecules. Thanks.
I’m not sure what you’re asking. Can you upload an image and indicate what you want?
We’re not a 2D chemical drawing program. So you want to hide the atoms somehow?
As I said, I’m not quite sure where the “condensed nitro” is supposed to be used in Avogadro.
Thanks
Actually I want to hide the bonds; that’s condensed formula. Just like describing benzene as C6H6, instead of drawing a hexagon with three C=C double bonds and six C-H bonds.
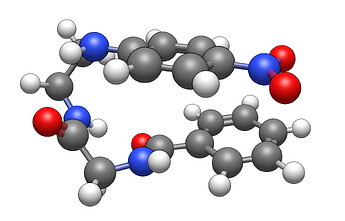
This is an example render from Avogadro including both a benzene and a nitro. What exactly do you want to see?
Jeff I think that we have a misunderstanding. It’s explained in the attachment that I sent you: a representation of a nitro group which does not include bonds: NO2 (lower case 2), just like a carboxylic acid, CO2H. Yes, show simple bonds if that is relevant, but otherwise they just get in the way. 99% of all chemists will agree with that statement.
I gave you benzene as an example, because a chemist will just write “C6H6” and not draw out bonds.
Sincerely
Fenton Heirtzler
Yes, we clearly have a misunderstanding.
Avogadro is a 3D molecular editor / viewer. The point is specifically to show and understand the 3D representation of molecules.
You’re asking about a feature to “condense” functional groups, which is used in 2D structure depiction to simplify a chemical drawing. The point is “oh, over here there’s something that you can expand in your head.”
That’s diametrically opposite the concept of a 3D viewer like Avogadro. There is literally no 3D viewer with that feature because the point is to show atoms (functional groups) where they exist in 3D space. For example, I can’t show a racemic chiral center (a 2D depiction) because in 3D you literally have to pick a stereochemistry.
In a 2D depiction, you might hide a nitro group because you want the viewer to concentrate on the rest of the molecule.
But the point of 3D depiction is that the spatial orientation and position of atoms is incredibly important.
The position and orientation of a nitro or benzene ring really matter. You can’t just replace them with a text label or sphere in a 3D depiction. You need to see the π-π stacking or the specific plane of the NO2 atoms.
Take a look at the image above. You’re asking for a feature in which you literally hide the terminal NO2 and slap a text label there. Or replacing the bottom benzene ring with a C6H6 label. I’m not even sure how the program would do that.
I have literally never seen a 3D representation image of a molecule anywhere with such text abbreviations. IMHO, the point of a 3D representation is to show the atoms in space. If you slap a “C6H6” in place of that benzene, you wouldn’t see that the benzene is pointed backwards from the nitro.
When I asked you for an image representing the feature you want, you have consistently used 2D chemical figures. That’s because the point of a 2D chemical figure is a stylistic representation of the molecule. The point of a 3D chemical figure is to literally show the atoms in their 3D positions. These are different goals. You haven’t given any indication of how your proposed “condensed formula” would work in a 3D depiction.
To me, these are entirely different conceptions of “how to show a molecule” and the whole point of a 3D molecular viewer like Avogadro.
Hi Geoff, I am indeed interested in looking 3D-representations of certain molecules, especially π-stacking, steric interactions and the means of illustrating their interactions. Not all big(ger) molecules are peptides, but can be complicated for the reader to appreciate. The nitro groups are important, but there is no need to show bonds, any more than other basic details.
What I finally did was to use “dummy” bromine atoms which can be updated in a graphics program as later on. If curious, then please see the attachment.
Bye
Fenton
(attachments)
@Fenton If
there is no need to show bonds, any more than other basic details.
I would propose to toggle off the display of bond orders and let the atoms to be connected by simple struts/single bond-like, just as in ORTEP-plots or the (supra)molecular graphs as popularized by Etter and Bernstein (example, example) – though I’m not aware if one can toggle-off all hydrogens except those required to depict chains and cycles between hydrogen bond donors and acceptors for visual clarity of the representation.
Hi, thanks for the suggestion. I’ve tried two different strategies:
- substituting Br for NO2, optimizing and editing with a graphics program (yes, it’s slimy, but at the minimum I just need some easy way of depicting complicated molecular structures.
- Graft the full NO2 group out of your “fragment” folder for nitrobenzene into the quinoxaline ring fragments, optimize (ongoing) and then surgery via a graphics program. It seems to be minimizing correctly.
Fenton